The nuclear envelope does not create the same topological barrier as most other membrane-bound organelles.
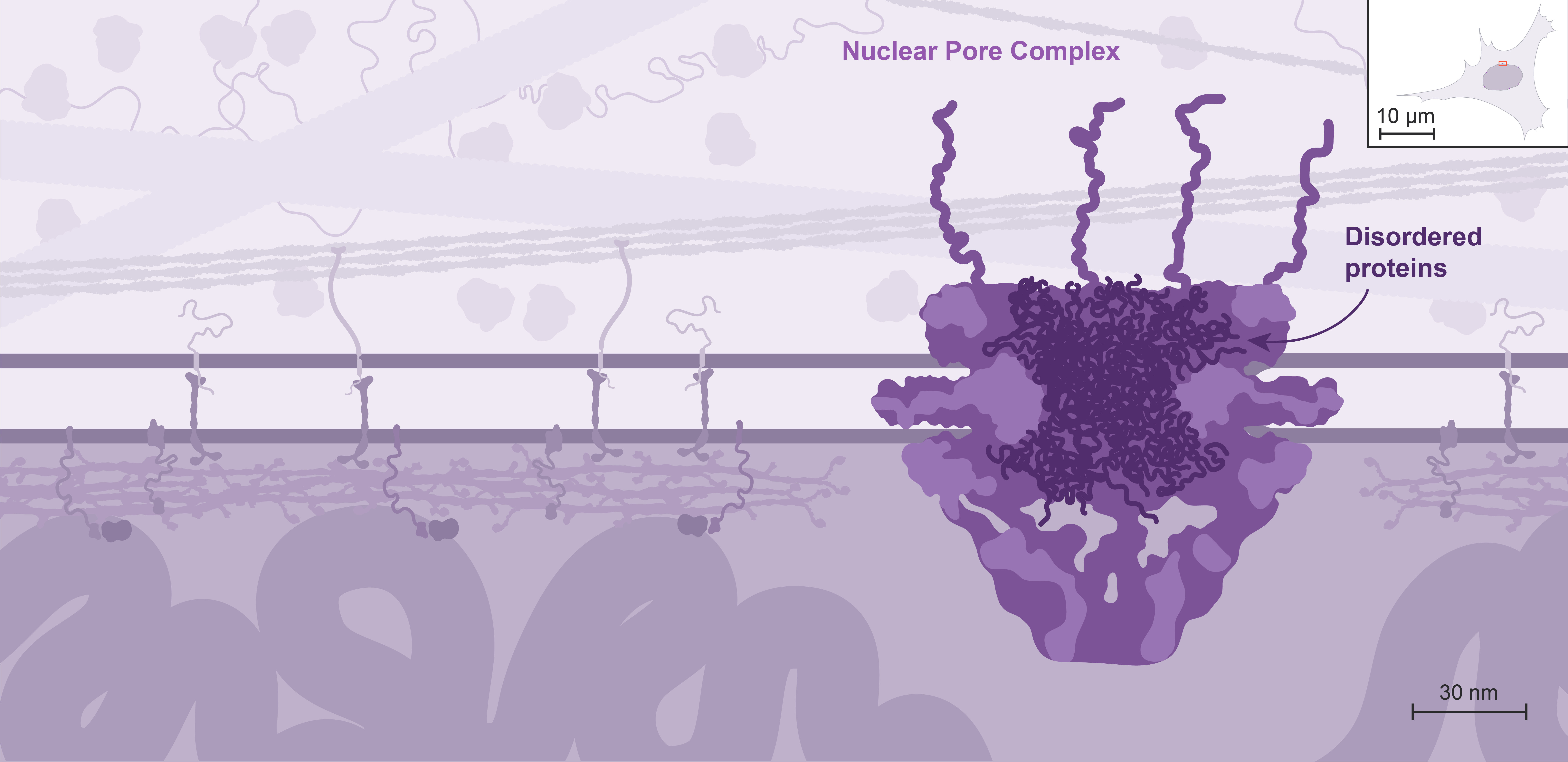
Instead, exchanges between the nucleus and the cytoplasm take place through large multiprotein channels called Nuclear Pore Complexes, or NPCs.
The nuclear envelope of a typical mammalian cell contains between 3000–4000 NPCs.
Mouse over the cell to explore the various organelles.
The nuclear envelope consists of two layers of membrane, enclosing a lumen, and is continuous with the endoplasmic reticulum.
The inner and outer nuclear membranes connect at the sites of the NPCs.
Mouse over the scene to explore all of its elements.
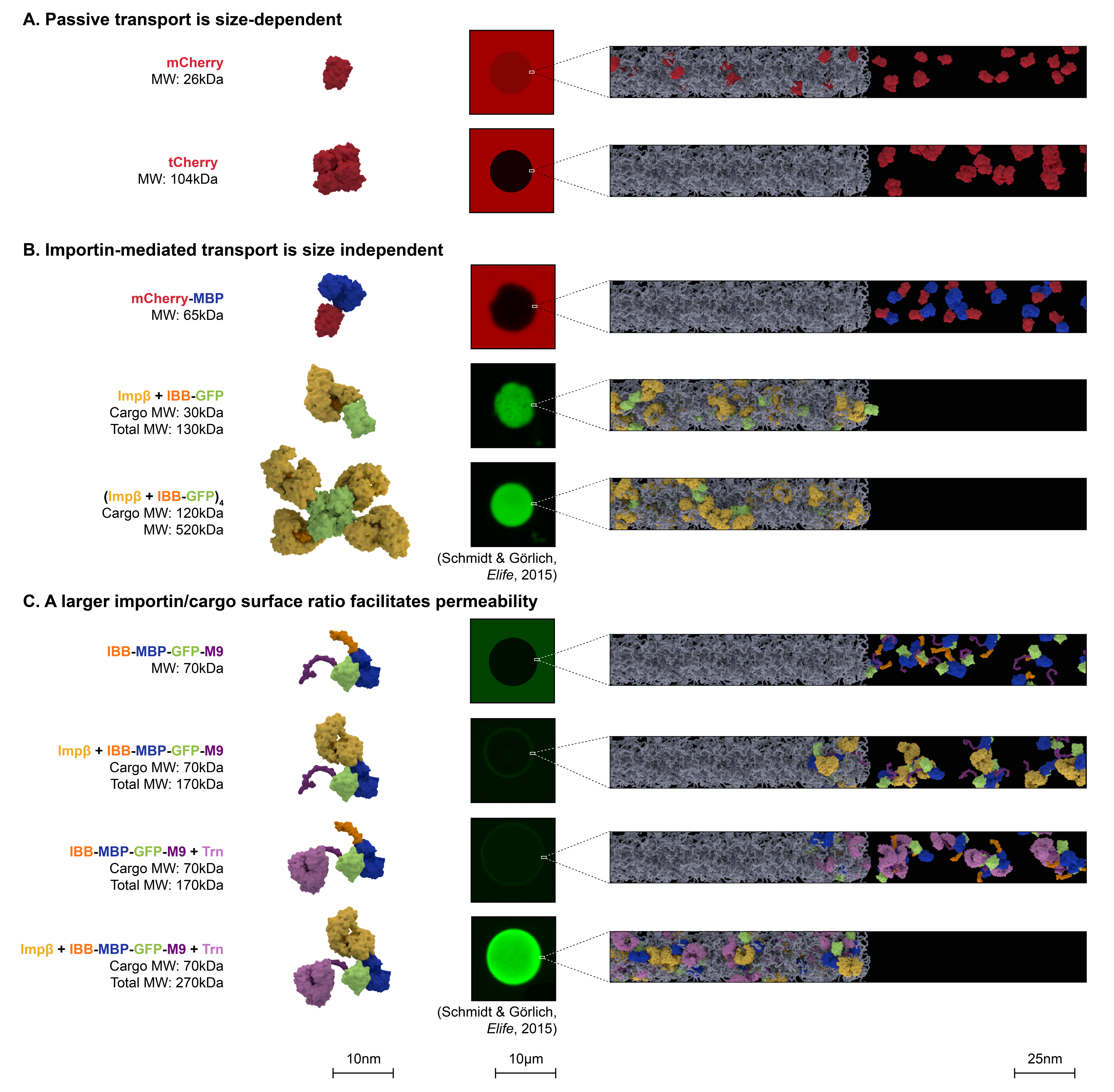
NPCs allow free passage of small molecules through diffusion, but regulate the transit of macromolecules as disordered proteins located within the central channel form a sieve that most macromolecules can’t cross by themselves.
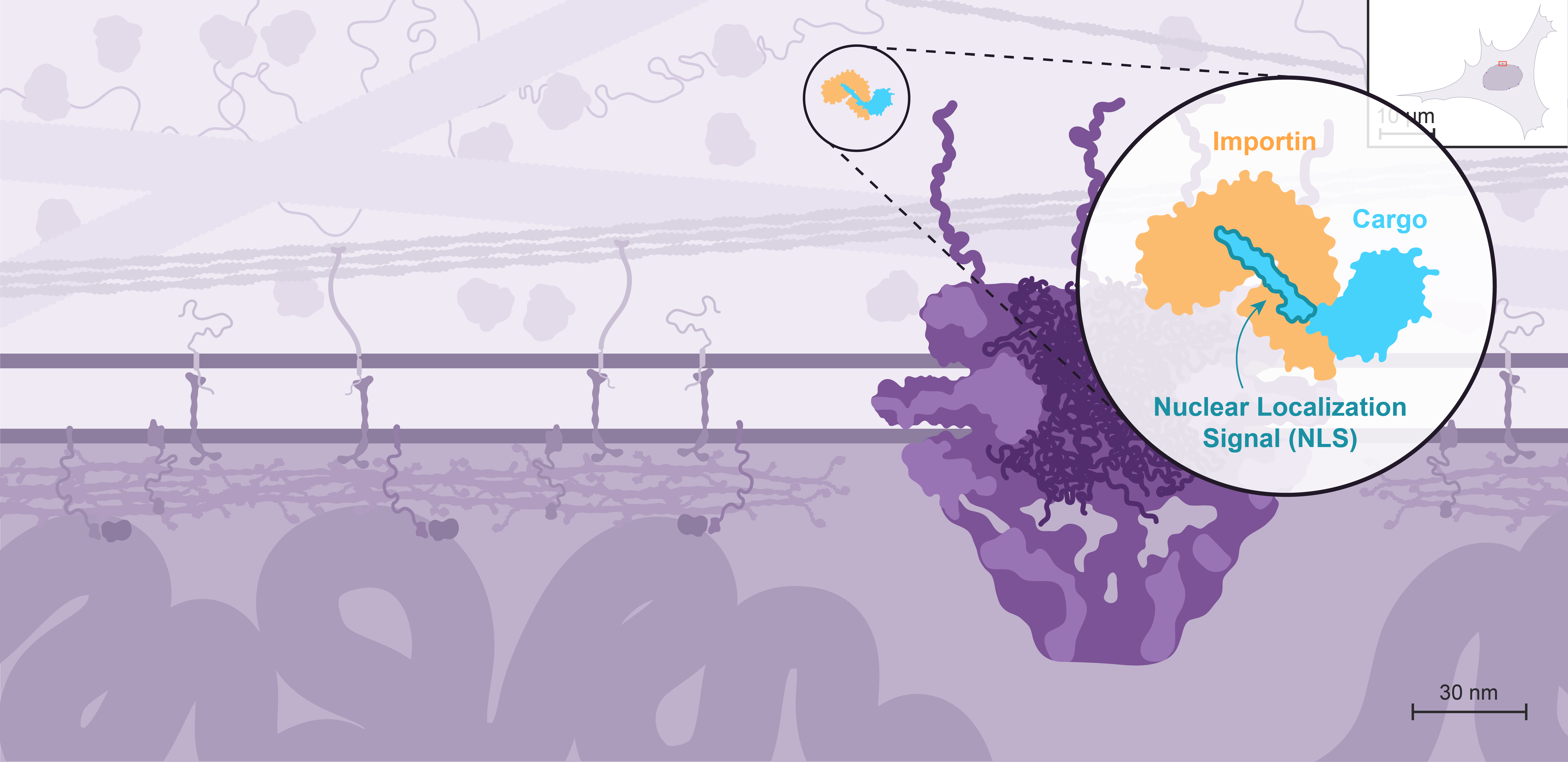
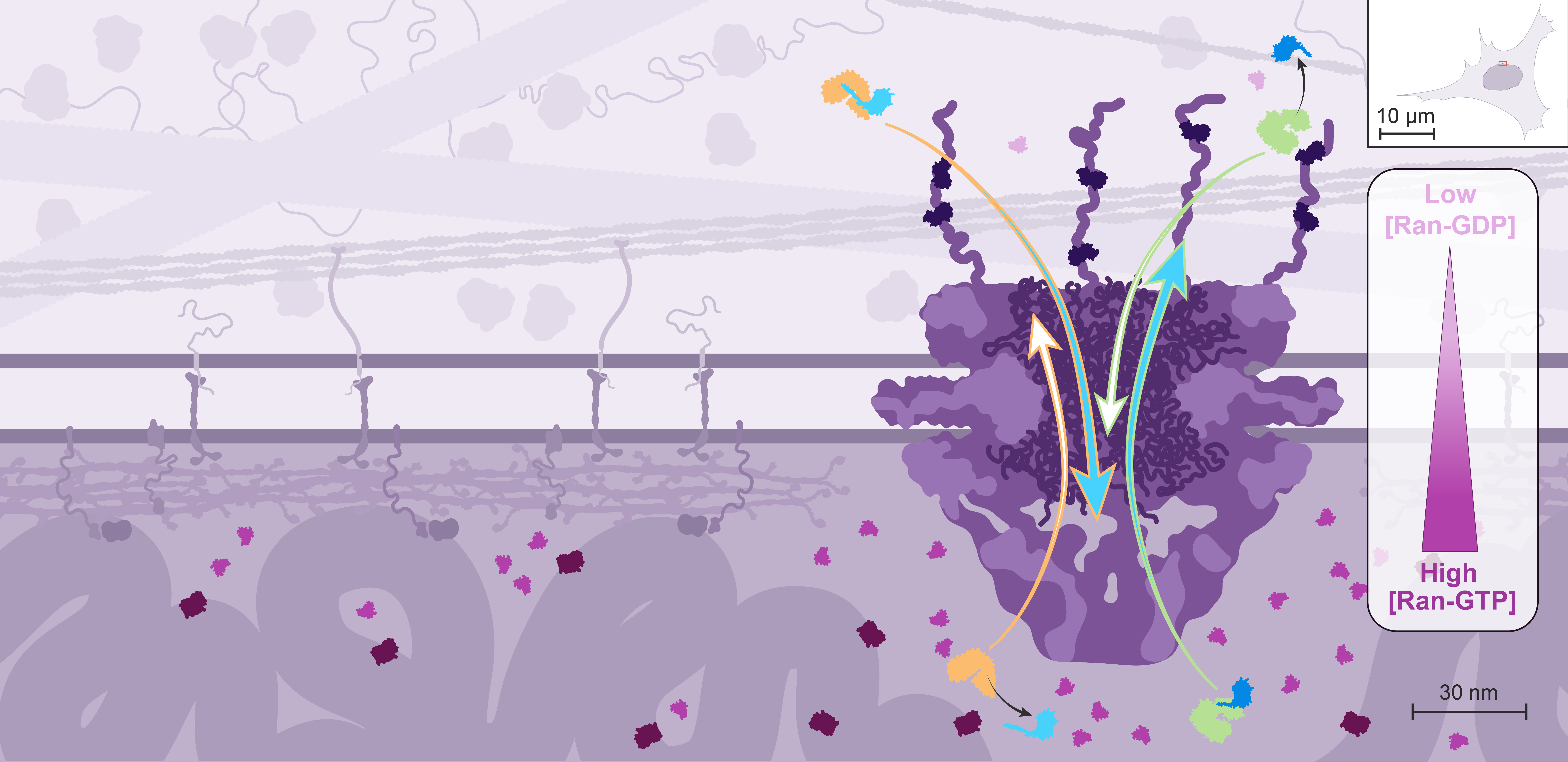
Nuclear import is mediated by transport receptors called importins, which recognize proteins containing a Nuclear Localization Signal, or NLS.
All the nuclear proteins - including structural organizers of the genome, transcription factors, and enzymes acting on DNA - are made in the cytoplasm and therefore need to get imported.
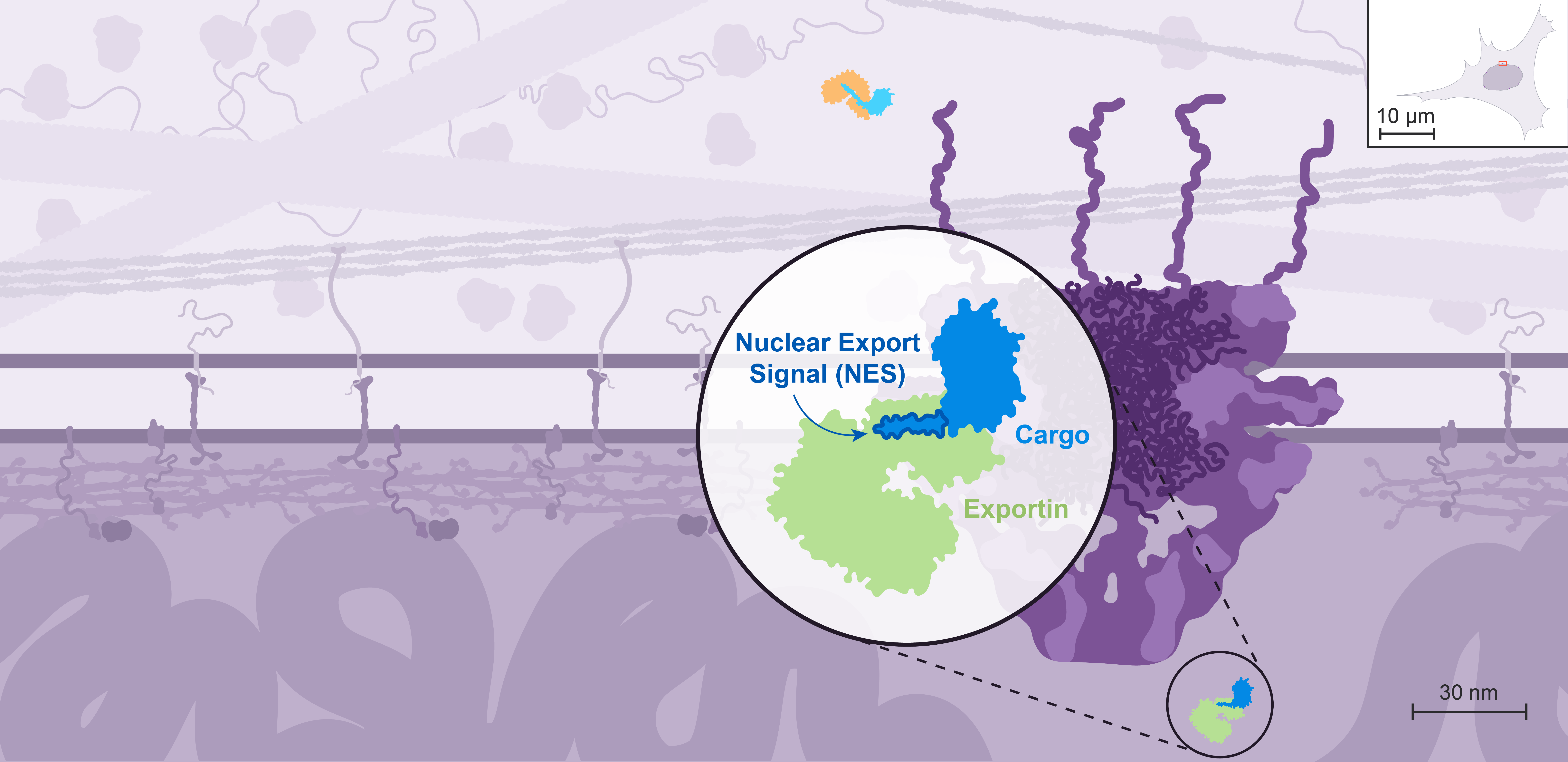
Nuclear export is mediated by different transport receptors called exportins, which recognize proteins containing a Nuclear Export Signal, or NES.
mRNA and ribosomes are examples of some of the elements that need to get exported.
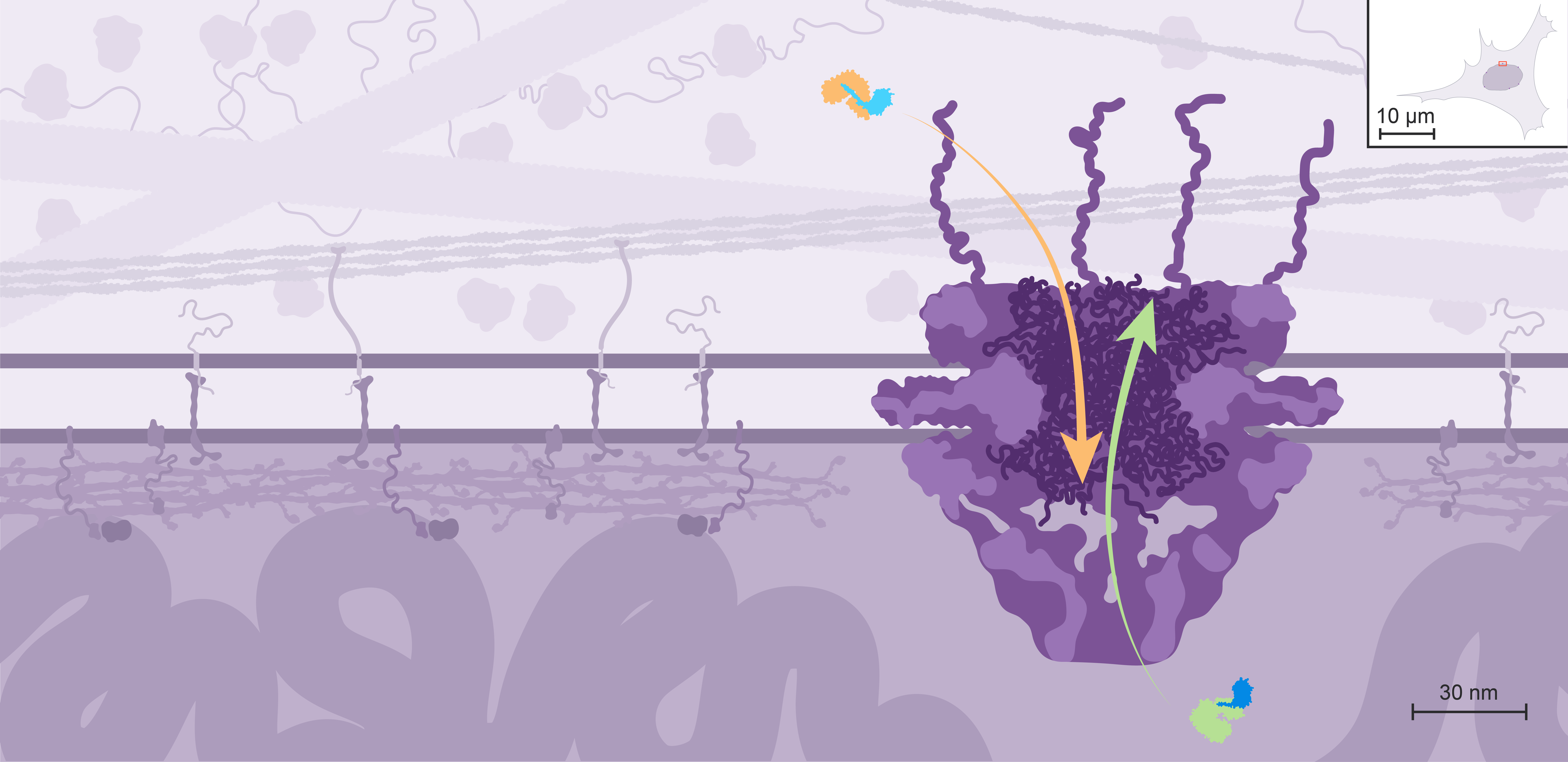
Importins and exportins weakly interact with the disordered proteins in the central channel. This facilitates the passage of these transport receptors and their cargo through the NPC. Even giant cargo, such as ribosomes, can be transported through the NPC in only a few milliseconds.
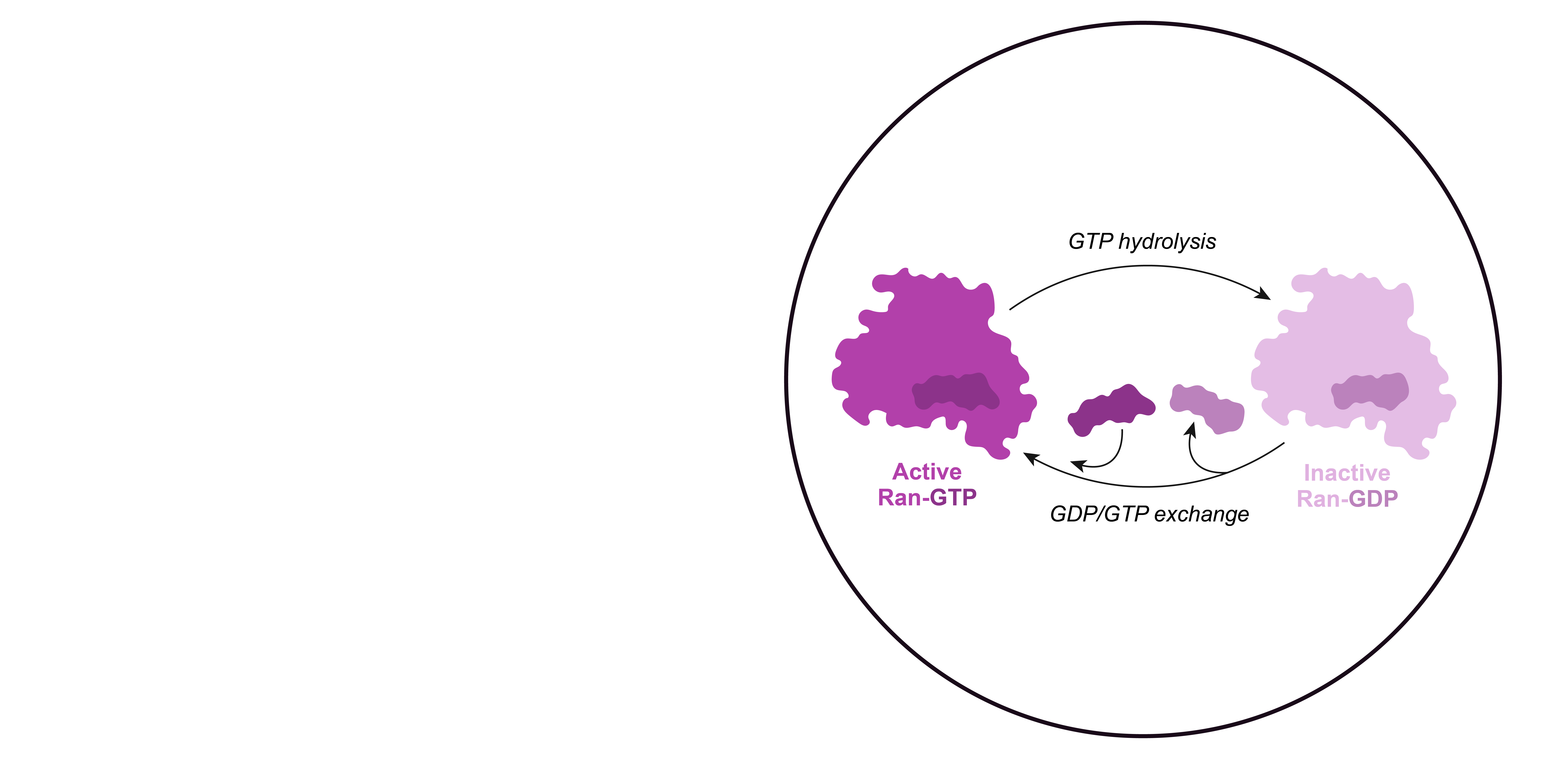
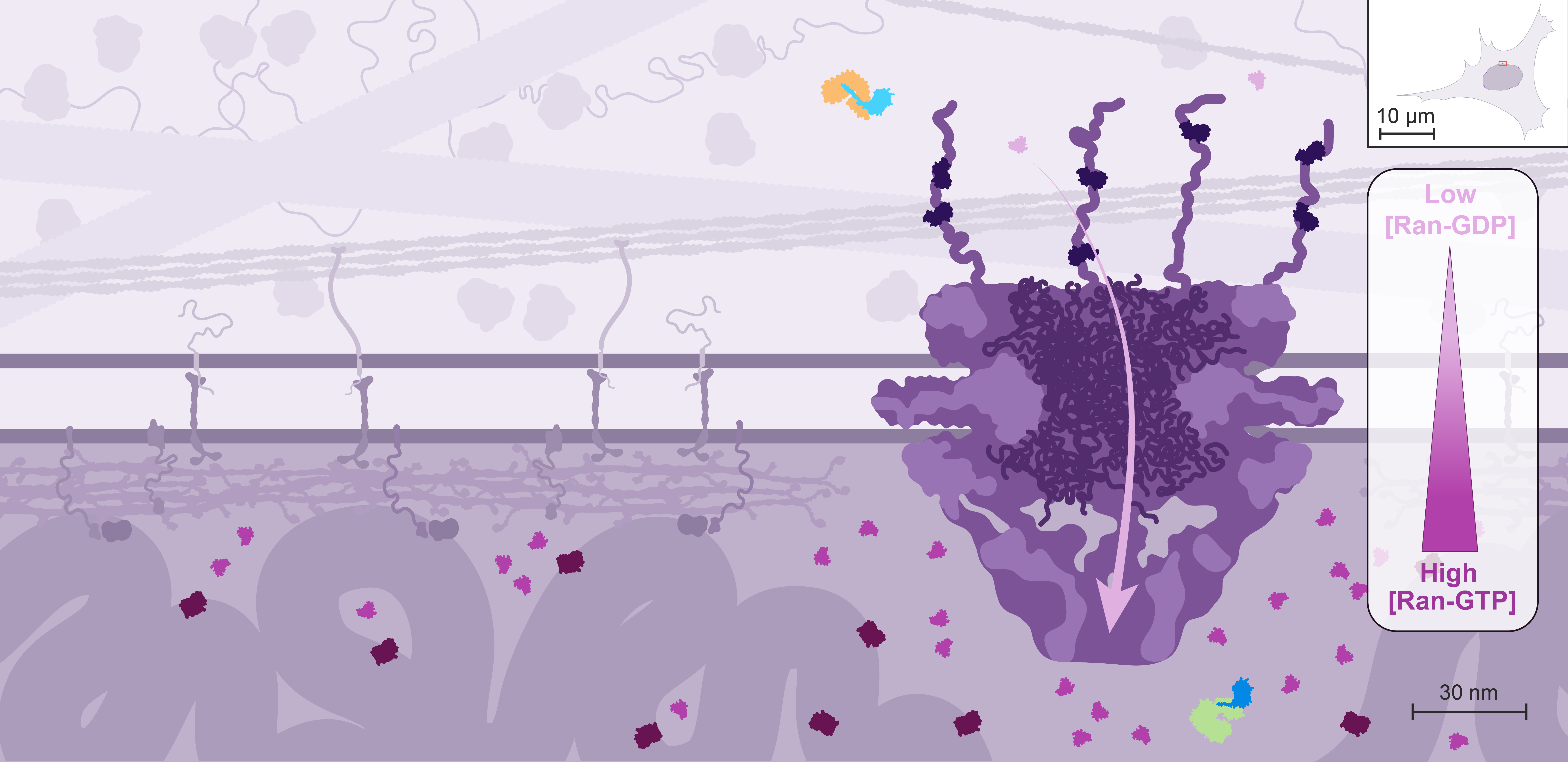
However, these random events cannot explain the directionality of nucleocytoplasmic transport, which can efficiently concentrate or exclude certain proteins. In this case, directional transport depends on the asymmetric distribution of the small GTPase Ran rather than on protein pumps.
Small GTPases like Ran are active when bound to GTP, and inactivate upon GTP hydrolysis. The GDP can be then replaced by another GTP.
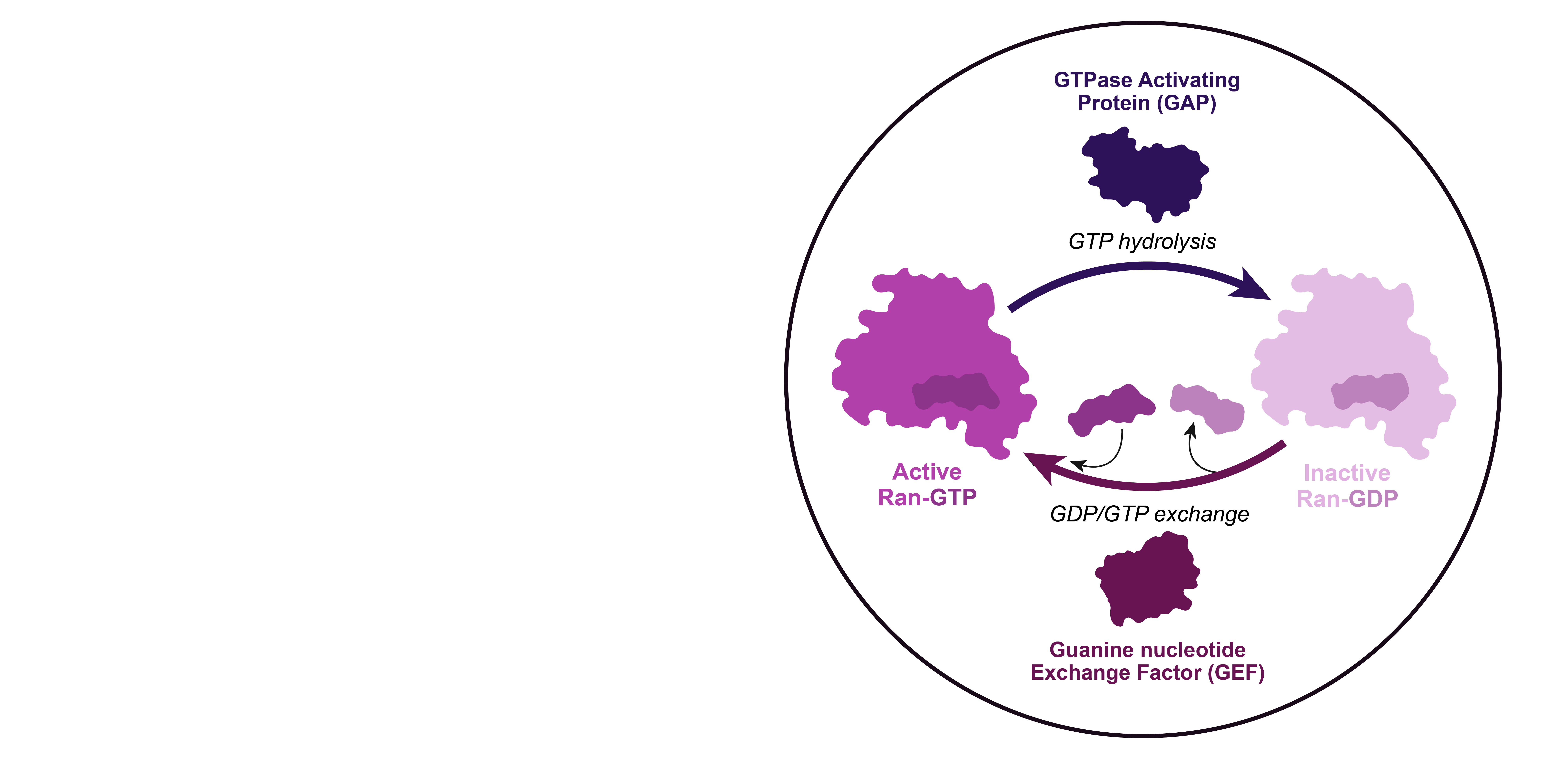
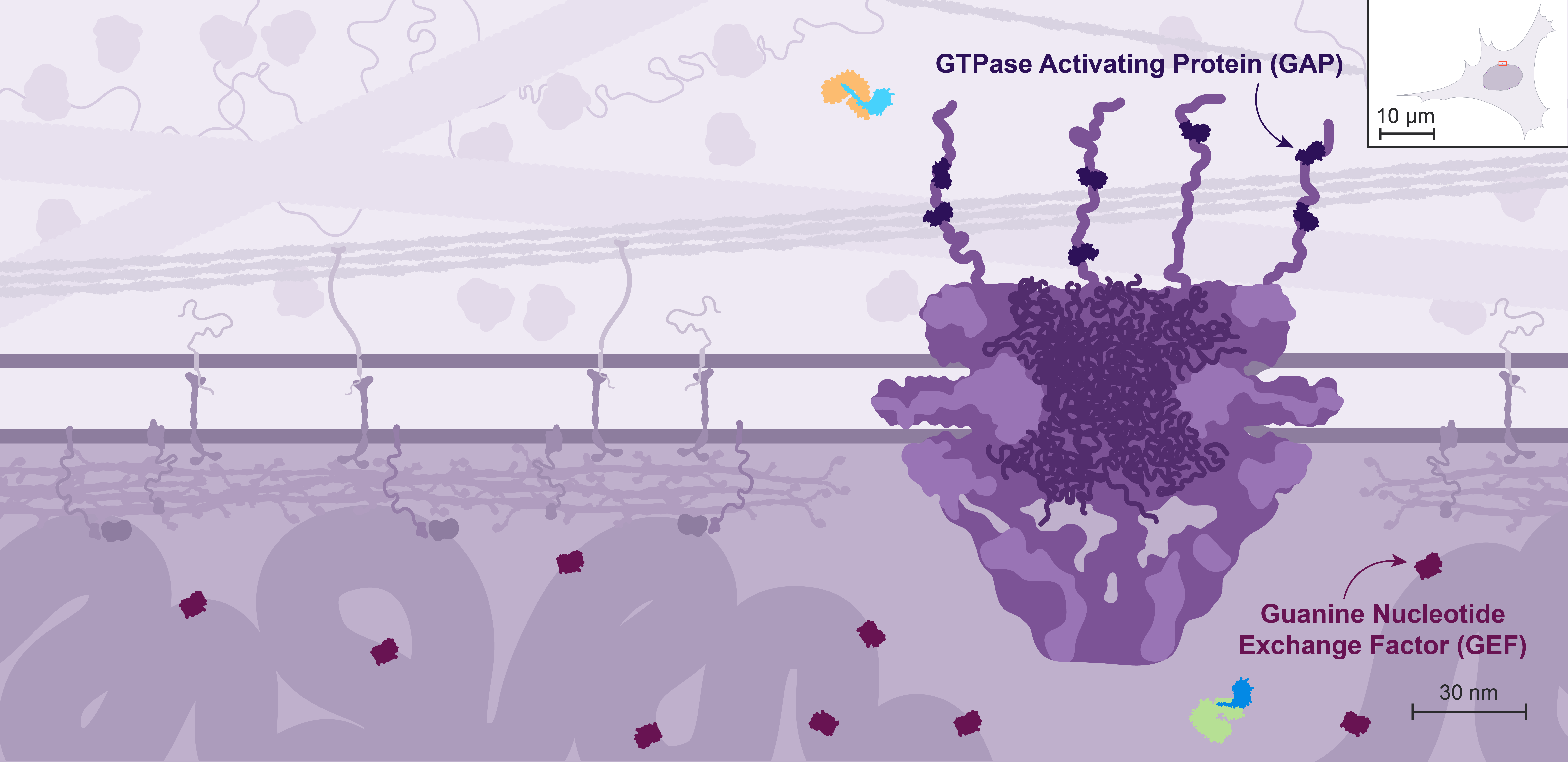
GTP hydrolysis by Ran is enhanced by GTPase Activating Proteins, or GAPs, while GTP exchange is promoted by Guanine nucleotide Exchange Factors, or GEFs.
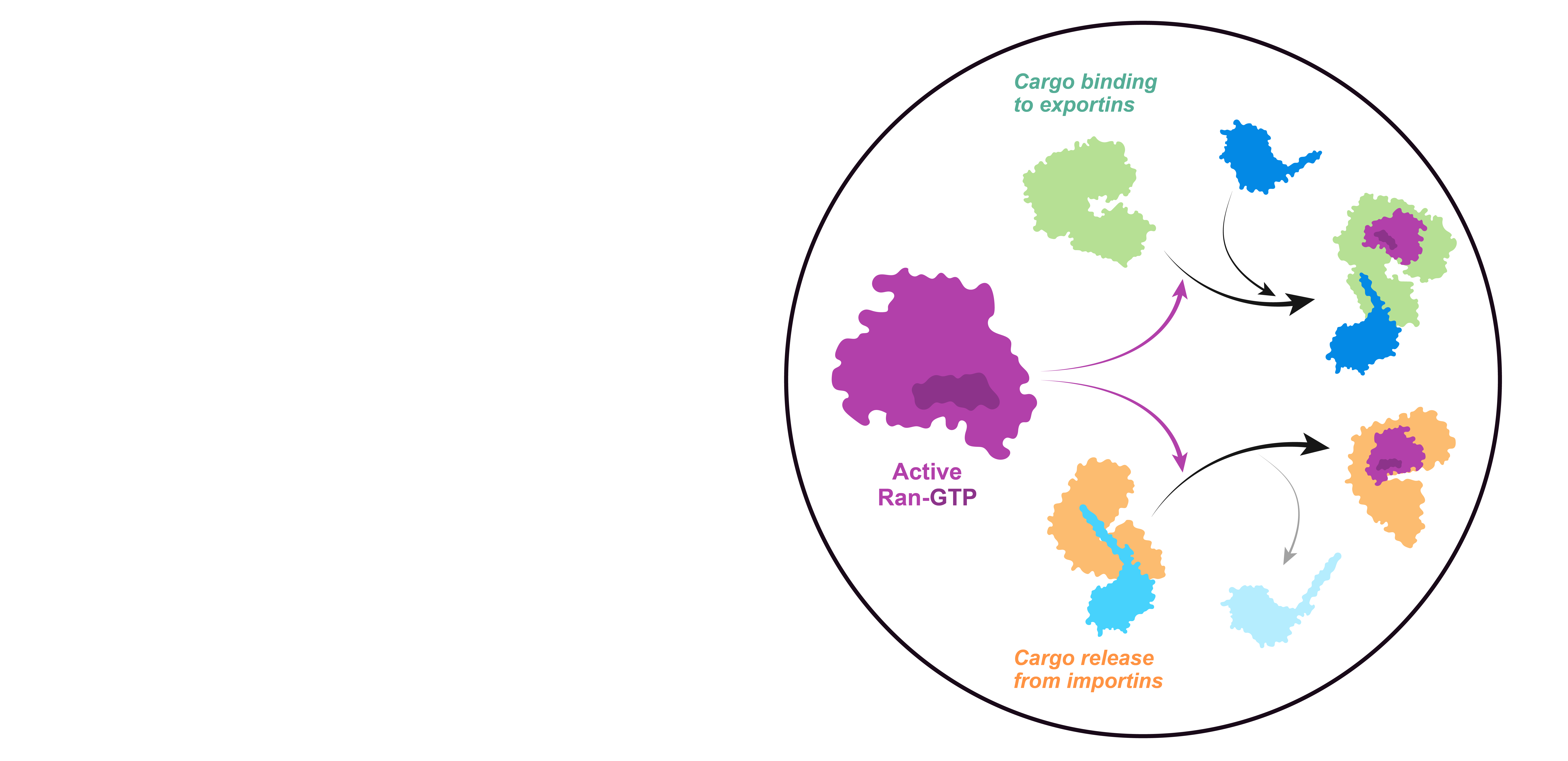
Only the GTP-bound form of Ran can interact with high affinity with both importins and exportins, but with opposite effects on cargo binding: it competes with cargos for importin binding but promotes their binding to exportins.
In the cell, the localization of Ran GEFs and GAPs is compartmentalized: the GEFs localize to the nucleus through association with the chromatin, while the GAPs are localized to the cytoplasmic side through interactions with components of the NPC.
As a result, Ran is predominantly loaded with GTP in the nucleus, and rapidly converted into the GDP-bound state in the cytoplasm. Ran-GDP is small enough to diffuse back passively into the nucleus, but its transport can also be facilitated by its own importin.
It is estimated that Ran-GTP is 200-fold enriched in the nucleus.
This Ran gradient controls the direction of nuclear import and export.